
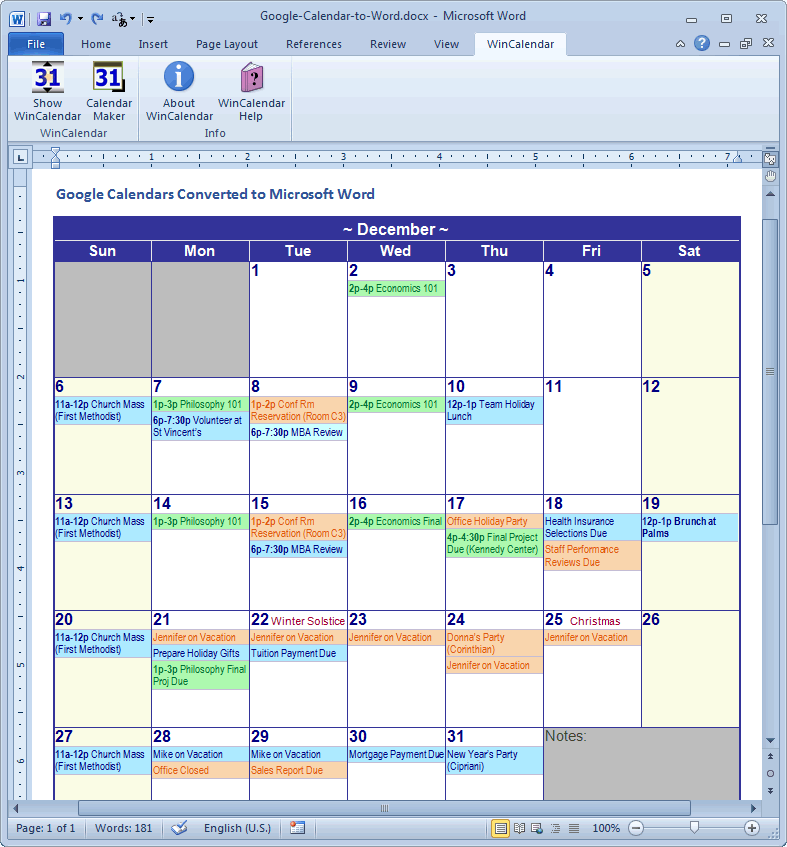
As the Agency provides relevant information and guidance for companies and individuals involved in developing and marketing medicines for human use in the Union no dedicated chapter on the centralised procedure has been included in the NTA. The European Medicines Agency is responsible for the scientific evaluation of applications for European Union (EU) marketing authorisations for human and veterinary medicines in the centralised procedure.

This Notice has no legal force and does not necessarily represent the final views of the Commission. The Notice to Applicants below has been prepared by the European Commission, in consultation with the competent authorities of the Member States and the European Medicines Agency (EMA). Volume 2 of the publications "The rules governing medicinal products in the European Union" contains a list of regulatory guidelines related to procedural and regulatory requirements such as renewal procedures, dossier requirements for Type IA/IB variation notifications, summary of product characteristics (SmPC), package information and classification for the supply, readability of the label and package leaflet requirements.


 0 kommentar(er)
0 kommentar(er)
